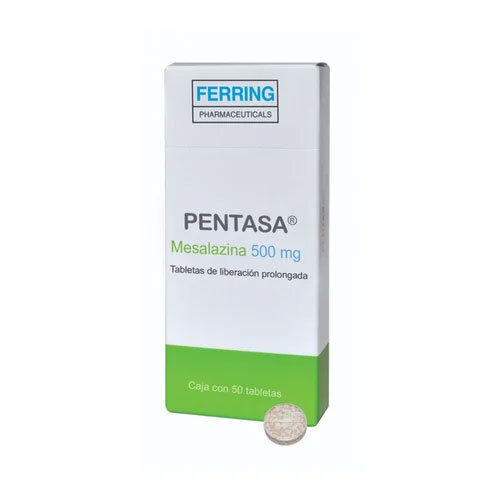
Pentasa 500 Mg Tab Mesalazine
Product Details:
- Dosage Form Tablet
- Pacakaging (Quantity Per Box) 100 tablets per box
- Salt Composition Mesalazine (5-Aminosalicylic Acid)
- Indication Treatment of Inflammatory Bowel Diseases (e.g. Ulcerative Colitis Crohns Disease)
- Origin of Medicine Synthetic
- Dosage 500 mg per tablet
- CAS No 89-57-6
- Click to view more
X
Pentasa 500 Mg Tab Mesalazine Product Specifications
- Synthetic
- Treatment of Inflammatory Bowel Diseases (e.g. Ulcerative Colitis Crohns Disease)
- Mesalazine (5-Aminosalicylic Acid)
- Pharmaceutical Drug
- Pharmaceutical Grade
- 2 years from the date of manufacture
- White or off-white round tablets
- Oral administration as prescribed by a healthcare provider
- 99% (Minimum Purity)
- C7H7NO3
- Tablet
- 89-57-6
- 500 mg per tablet
- Store below 25°C in a dry place away from direct sunlight
- 100 tablets per box
Product Description
Pentasa 500 Mg Tab Mesalazine is a pharmaceutical drug used for the treatment of Inflammatory Bowel Diseases such as Ulcerative Colitis and Crohns Disease. Each tablet contains 500 mg of Mesalazine (5-Aminosalicylic Acid) and comes in white or off-white round tablets. The minimum purity of the medicine is 99% and it is stored below 25C in a dry place away from direct sunlight. Manufactured by Ferring Pharmaceuticals, Pentasa is available in a packaging size of 100 tablets per box, with each box containing 1*10 tablets.
| Strength | 500 mg |
| Manufacturer | Ferring Pharmaceuticals |
| Brand | Pentasa |
| Packaging Size | 100 Tablets Pack |
| Packaging Sizes | 1*10 Tablets |
| Packaging Type | Box |
FAQs of Pentasa 500 Mg Tab Mesalazine:
Q: What is the strength of each Pentasa tablet?
A: Each Pentasa tablet contains 500 mg of Mesalazine.Q: How should Pentasa be stored?
A: Pentasa should be stored below 25C in a dry place away from direct sunlight.Q: Who is the manufacturer of Pentasa?
A: Pentasa is manufactured by Ferring Pharmaceuticals.Q: What is the indication for Pentasa?
A: Pentasa is indicated for the treatment of Inflammatory Bowel Diseases like Ulcerative Colitis and Crohns Disease.Q: What is the expiration date of Pentasa tablets?
A: The expiration date of Pentasa tablets is 2 years from the date of manufacture.Other Products in 'Anti Infective Medicine' category
Get in touch with us