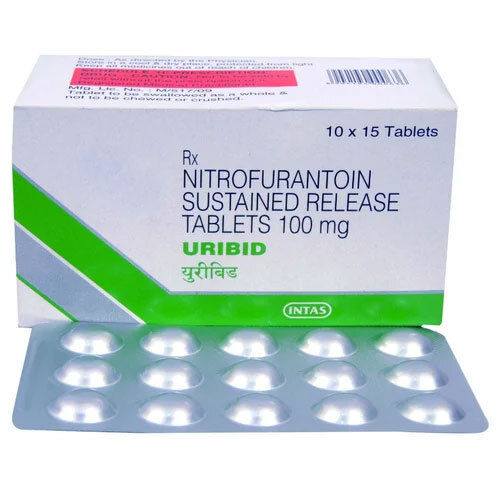
Nitrofurantoin Sustained Release 100 Mg Tab
Product Details:
- Dosage Form Tablet
- Origin of Medicine Synthetic
- Expiration Date 2 years from the date of manufacture
- Salt Composition Nitrofurantoin (sustained release)
- Dosage 100 mg
- Grade Pharmaceutical Grade
- Storage Store in a cool and dry place protected from light and moisture
- Click to view more
X
Nitrofurantoin Sustained Release 100 Mg Tab Product Specifications
- Yellow circular and film-coated tablet
- 10 x 10 Tablets
- Treatment of urinary tract infections
- Prescribed for bacterial infections of the urinary tract
- 67-20-9
- C8H6N4O5
- Antibiotic
- 98.0% - 102.0% (on anhydrous basis)
- Store in a cool and dry place protected from light and moisture
- Pharmaceutical Grade
- 100 mg
- Nitrofurantoin (sustained release)
- 2 years from the date of manufacture
- Synthetic
- Tablet
Product Description
Nitrofurantoin Sustained Release 100 mg Tab is a synthetic antibiotic prescribed for bacterial infections of the urinary tract. Each yellow circular and film-coated tablet contains Nitrofurantoin (sustained release) as the active ingredient. The medicine comes in a packaging of 10 x 10 tablets, with a dosage of 100 mg per tablet. It has a shelf life of 2 years from the date of manufacture and should be stored in a cool, dry place protected from light and moisture. Nitrofurantoin Sustained Release 100 mg Tab is a pharmaceutical-grade drug with an assay of 98.0% - 102.0% on an anhydrous basis.
| Strength | 100 mg |
| Packaging Type | Strips |
| Packaging Size | 1*10 Capsule |
| Brand | Uribid |
| Manufacturer | Intas Pharmaceuticals Ltd. |
| Shelf Life | 24 months |
FAQs of Nitrofurantoin Sustained Release 100 Mg Tab:
Q: What is the strength of Nitrofurantoin Sustained Release 100 mg Tab?
A: The strength of each tablet is 100 mg.Q: What is the recommended dosage for this medicine?
A: The recommended dosage is 100 mg per tablet.Q: What is the expiration date of Nitrofurantoin Sustained Release 100 mg Tab?
A: The expiration date is 2 years from the date of manufacture.Q: What is the storage requirement for this medicine?
A: It should be stored in a cool and dry place protected from light and moisture.Q: What is the indication for using Nitrofurantoin Sustained Release 100 mg Tab?
A: It is indicated for the treatment of urinary tract infections.Other Products in 'Antibiotic Medicine' category
Get in touch with us