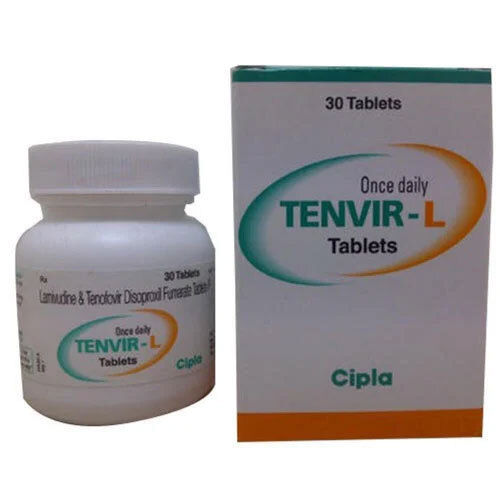
Lamivudine And Tenofovir Disoproxil Fumarate Tablets
Product Details:
- Usage Taken orally as prescribed by a healthcare professional
- Appearance White to off-white tablets
- Expiration Date 24 months from the date of manufacture
- Storage Store below 30°C protect from light and moisture
- CAS No 134678-17-4 (Lamivudine) 202138-50-9 (Tenofovir Disoproxil Fumarate)
- Assay Not less than 98% purity for both active ingredients
- Molecular Formula Lamivudine (C8H11N3O3S) Tenofovir Disoproxil Fumarate (C19H30N5O10P·C4H4O4)
- Click to view more
X
Lamivudine And Tenofovir Disoproxil Fumarate Tablets Product Specifications
- 134678-17-4 (Lamivudine) 202138-50-9 (Tenofovir Disoproxil Fumarate)
- White to off-white tablets
- Taken orally as prescribed by a healthcare professional
- 24 months from the date of manufacture
- Store below 30°C protect from light and moisture
- Synthetic
- Lamivudine 300 mg + Tenofovir Disoproxil Fumarate 300 mg per tablet
- Used for the treatment of HIV-1 infection in adults and adolescents
- Not less than 98% purity for both active ingredients
- Lamivudine (C8H11N3O3S) Tenofovir Disoproxil Fumarate (C19H30N5O10P·C4H4O4)
- 30 tablets per box
- Antiretroviral
- Pharmaceutical Grade
- Tablets
- Lamivudine Tenofovir Disoproxil Fumarate
Product Description
Lamivudine and Tenofovir Disoproxil Fumarate Tablets are pharmaceutical-grade antiretroviral tablets used for the treatment of HIV-1 infection in adults and adolescents. Each tablet contains Lamivudine 300 mg and Tenofovir Disoproxil Fumarate 300 mg. The white to off-white tablets have a purity of not less than 98% for both active ingredients. These tablets are taken orally as prescribed by a healthcare professional and come in a packaging size of 30 tablets per box with an expiration date of 24 months from the date of manufacture. The salt composition includes Lamivudine and Tenofovir Disoproxil Fumarate, with a synthetic origin.
| Packaging Size | 30 Tablets Bottle |
| Brand | Tenvir L |
| Manufacturer | Cipla Ltd |
| Composition | Lamivudine (300mg) + Tenofovir disoproxil fumarate (300mg) |
| Treatment | HIV / AIDS |
| Prescription/Non prescription | Prescription |
FAQs of Lamivudine And Tenofovir Disoproxil Fumarate Tablets:
Q: What is the dosage form of Lamivudine and Tenofovir Disoproxil Fumarate Tablets?
A: The dosage form of Lamivudine and Tenofovir Disoproxil Fumarate Tablets is in the form of tablets.Q: What is the recommended dosage of these tablets?
A: The recommended dosage is Lamivudine 300 mg + Tenofovir Disoproxil Fumarate 300 mg per tablet.Q: How should these tablets be stored?
A: These tablets should be stored below 30C, protected from light and moisture.Q: What is the indication for using Lamivudine and Tenofovir Disoproxil Fumarate Tablets?
A: These tablets are used for the treatment of HIV-1 infection in adults and adolescents.Q: Who is the manufacturer of Tenvir L tablets?
A: Tenvir L tablets are manufactured by Cipla Ltd.Other Products in 'HIV Medicine' category
Get in touch with us